Tracing the Sounds of the Lungs

The Geneva-based start-up Onescope is developing an AI-powered device for automatically analysing respiratory sounds.
The stethoscope is the number one tool for every doctor. Many medical consultations begin with an obligatory check of heart and lung sounds using this small diagnostic instrument. It is indispensable and central to medical examinations, and with good reason. Worldwide, pneumonia is responsible for 14% of all deaths among children under five. According to the World Health Organization (WHO), more than 700,000 people die from it every year.
Inspired by the music recognition app Shazam
Since 2016, Alain Gervaix, a paediatrician at the Geneva University Hospitals (HUG) children’s hospital in Switzerland, has been exploring how the stethoscope could be improved, particularly in regions where trained medical staff are scarce. The idea came from his daughter, who asked why it shouldn’t be possible to identify certain patterns in lung sounds using AI, much like Shazam recognises songs.
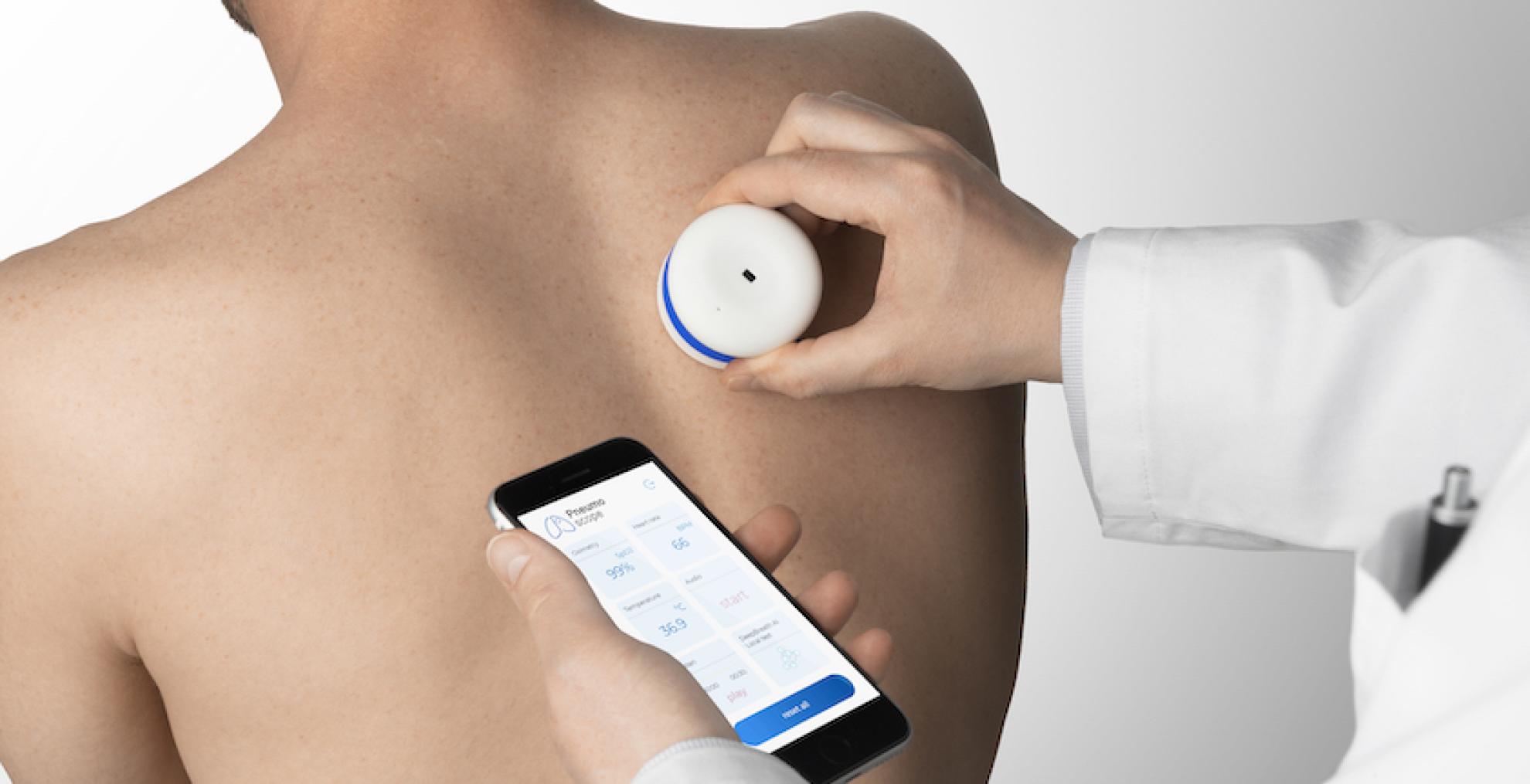
Thanks to AI and extensive reference data, Onescope can immediately provide initial findings when listening to breathing sounds.
Diagnoses through the recording of breathing sounds
This concept resulted in the development of the Pneumoscope prototype, a compact device resembling a traditional stethoscope, but offering much more. Thanks to a collaboration with EPFL it can measure oxygen saturation and body temperature, and connect to an app that analyses all the data. The principle is elegantly simple: the device records breathing sounds, which the AI then compares with thousands of reference patterns to provide an initial assessment within seconds.
250,000 data samples
The underlying database is enormous, with over 250,000 data sets from Switzerland, the Democratic Republic of the Congo, Mozambique, and other countries incorporated into the system over the past four years. The advantages of such an automated diagnostic tool are particularly evident in remote regions with limited medical infrastructure, where local health workers can determine whether a patient needs to be transferred to hospital or can be treated on-site with minimal training.
Clinical studies are paving the way for approval
To bring the prototype to market, the start-up Onescope was founded last year and has since raised around CHF 3 million in grants and awards. With a small but highly specialised team, Onescope is currently conducting clinical trials, primarily in Mozambique and the Democratic Republic of the Congo. The goal is to obtain CE certification by the end of 2025, which would be a decisive step towards market approval in Europe. FDA approval for the United States is expected to follow.




